Describe the Movement of the Particles in the Air.
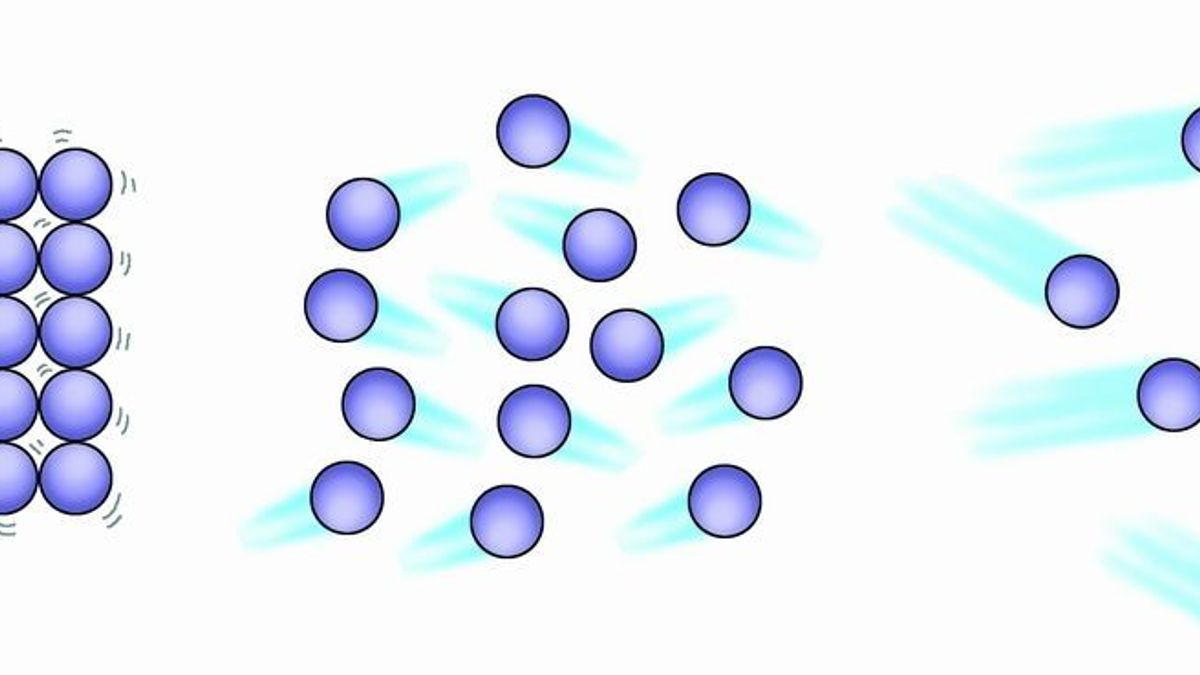
They move in random directions. 101 understand the three states of matter in terms of the arrangement movement and energy of the particles.
They reach a similar point and come together as the wave reaches the highest point and then drift separate at the lowest point.
. Some longitudinal waves are. The movement of air is mainly caused by the differences in pressure and temperature. The motion of all the particles is predictable.
Kinetic energy increases It could be dangerous if the temperature of the air inside the canister increased by a large amount. They move in random directions. They move with a.
Once inside the nasal cavity the air passes through the nasal conchae. The motion of all the particles is predictable. The speeds of the particles vary but on average they move quicker than they do in.
Your child can examine the sticky areas each day with her magnifying glass. Name of Process Changes in Arrangement Movement and Energy Melting The particles gain kinetic energy to a point where they vibrate faster. Learn vocabulary terms and more with flashcards games and other study tools.
They move all around the wave. This phenomenon creates wind. Gases are made up of particles eg.
Warm air is lighter and it rises upwards meanwhile cold air is denser and hence it moves down to replace the warm air. The particles in a gas are moving very quickly in random directions. Another evidence for the movement of particles in liquids is when you.
Air from the surrounding area is sucked into the space left by the rising air. Start studying describe the movement of particles in solids liquids gases and plasma states. Mary put the fire out.
Figure 1 a Which two sentences describe the movement of the air particles in the canister. The names of the interconversions how they are achieved and the changes in arrangement movement and energy of the particles. They move in circular paths.
Explain that air has fine particles which cannot be seen without a powerful microscope. The term for a mixture of solid particles and liquid droplets found in the air. After 6 or 7 days have her compare which card collected more particles on the sticky surfaces.
As it travels the air makes rapid swirls of movement in order to cause small particles in the air to stick to mucus. They move with a range of different speeds. 1 Which TWO sentences describe the movement of the air particles in the canister.
PM stands for particulate matter also called particle pollution. The air cools until it descends. Horizontal flow is called advection.
Warm air rising creates a low pressure zone at the ground. Among all the forces acting on the particles will be the internal forces that the particles exert on one another. Up to 24 cash back First air enters your body either through your nose or your mouth where it is then held in your nasal cavityoral cavity.
Newtons third law says that all these internal forces act in pairs action and reaction. Others are so small they can only be detected using an electron microscope. The particles move in straight lines at different speeds.
What happens to the movement of the air particles. Movement of Air. Air flows horizontally at top of the troposphere.
There are attractive forces between particles. Turn over 20 20 BLANK PAGE 21 21. In liquids there is a mild force of attraction between the particles it holds them together but at the same time allow their free movmentwhile in a gasThere is no force of attraction between the particlesso they move randomly.
But she will have noticed other particles. This means that the movement of the medium is in the same direction as the motion of the wave. Mary put out it.
1 mark increases What is an alpha particle. Mary put out the fire. The particle movement transformation is optional in subject-verb-direct object sentences with particles except when the direct object the NP is a personal pronoun.
Let the cards sit for about a week. 2 marks Tick TWO boxes. Observe the motion of the three colored air particles.
Which makes you able to SMELL it. They move in the direction opposite to that of the wave. Theoretically then they cancel each other out in the vector sum.
They vibrate about a fixed position. Up to 24 cash back Some evidence for the movement of particles in gases is when the perfume gas molecules diffuse into the air when you put it on because the particles from the perfum diffuses with the gas particles around you and it spreads all over your room. Particles movement is determined by the energy they have and their relation to other particles.
They move in the same direction as the wave. -Imagine a box full of air particlesThe particles are forced to move to a point A on the edge of the boxMy question is nowhow can I mathematicly describe the. These particles are constantly moving because they have kinetic energy.
The size of these particles is very small compared to the distance between the particles. 2 marks it could explode What happens to the volume of the air when it is released from the canister. This allows the particles to overcome the forces of attraction in a solid which means the regular pattern is broken down and the particles can slide over each other.
Hello everybody I have a new thread to postit is very important to find a solution for this. Describe the motion of these particles for a sound wave moving through air. Which statement best describes the motion of air particles in a sound wave.
Some particles such as dust dirt soot or smoke are large or dark enough to be seen with the naked eye. In this case the transformation is obligatory. They vibrate about a fixed position.
102 understand the interconversions between the three states of matter in terms of. A few basic principles go a long way toward explaining how and why air moves.

Arrangement Of Particles In Phases Of Matter Comparison Expii
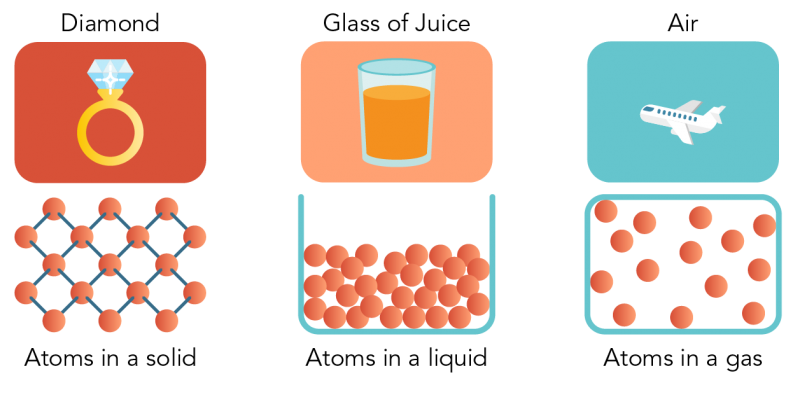
Introduction To The Particle Theory Of Matter Let S Talk Science

Comments
Post a Comment